Happy Friday! I’ve been catching up on my blog
reading. One of the blogs I regularly read is Bryan Alexander’s (author of Academia Next), and a couple of weeks
ago he posted a video and summary on the topic of curricular analytics with guest
presenter Gregory Heileman, who is both an administrator and an engineering
professor. The video and Q&A bring up many interesting points that
could fill multiple blog posts. Today I’ll just focus on one of those:
Curricular Complexity.
What is curricular complexity? (Watch the video!)
In a nutshell, it’s an ad hoc way of quantifying who complicated it is for a
student to go through the pathway of a major given prerequisites, corequisites,
and timing of the classes offered. Science and engineering majors, which tend
to be more hierarchical than humanities majors score high in curricular
complexity – and this has potential downsides. For one, there is a negative correlation
between the four-year graduation rate and curricular complexity. And once you
dig into the data you find all sorts of other interesting correlations, for
example, curricular complexity is inversely correlated with university
reputation (at least in electrical engineering). Hmmm… I’m sure you’re starting
to wonder why this might be.
Well, it’s complicated. Or I should say
multi-factorial. We could discuss elitism, student preparedness, selection
methods, accrediting bodies, history, narrow-minded professors, differences in
disciplines, education costs, laboratories, bottlenecks, weed-out courses, and
so much more. Many of these do not have easy answers and the relationships are
a tangled web. I’m not going to do that here. Instead I turn my eye to how the
curriculum in my department has changed over the years and consider some of the
factors underpinning its logic.
First, some background: I’ve been at my institution
for about twenty years and we’ve made a number of curricular changes over that
time period. I’m ensconced in a liberal arts college (with no graduate programs
in chemistry and biochemistry) focused on undergraduates. When I started we
averaged slightly less than twenty majors per year. These days we typically
have forty or so majors per year. When I started we just had a chemistry major
that included a biochemistry pathway. There were only minor differences between
the “straight” chem major and the biochem path. Three major factors have driven
our curricular changes: rising numbers of students in our classes, the design
of a new separate biochemistry major, and the addition of a research
requirement for our majors. Our chemistry majors are ACS-accredited, and our
biochemistry majors are ASBMB-accredited, i.e., our curriculum meets the
requirements of the professional bodies in chemistry and biochemistry
respectively.
In Heileman’s presentation (watch the video!),
curricula are analyzed as graph-networks. Turns out I’ve recently taught myself
the basics of network and graph theory for a grant proposal I just submitted,
so I’m reasonably versed. But you don’t need those details to get the presentation
(you won’t even notice the few non-crucial bits of jargon). I decided to draw
out our present curricula using the framework. I haven’t included the numerical
“complexity factor” because it’s not important unless you’re trying to compare
across a bunch of different curricula at a bunch of different schools.
Here’s our current chemistry major. I’ve slotted
things into semesters over a four-year plan (the large majority of our majors
graduate in four years) based both on the recommended pathway and what students
tend to do. Not being a large university, we do not have the bandwidth or
resources to offer every class every semester. While G-Chem 1, Biochem 1
lecture, Analytical chem, Research Methods, are offered every semester; the
other courses in our major are not. Both semesters of calculus and physics
(taught by other departments) are offered every semester; but Physics 1 is
offered mostly in the Spring and Physics 2 is offered mostly in the Fall, and I’ve
placed them when many of our students take them.

The two key things to think about in the graph are:
(1) What are the longest sequential paths through the network? (2) Where are
the bottlenecks? The longest pathway is five arrows tracing from G-Chem through
O-Chem to Inorganic to Advanced Synthesis. This means that technically a
student can finish the major in three years, although most do it in four. There
are two “hubs”: The key hub is G-Chem 2 which must be completed before getting
to any of the other classes. The secondary hub is O-Chem 2 which is a
prerequisite for Biochem 1 lecture, Inorganic and Advanced Synthesis. The
majority of our electives require O-Chem as a prerequisite, but not all.
We previously had prerequisites linking up P-Chem 1
with P-Chem 2 and Inorganic, but we removed these when we redesigned our new
biochemistry major. Along with this we streamlined our “senior” labs from four
rotating offerings (with different prerequisites) to just two that all our
chemistry majors must take. The overall effect of breaking these links is a
reduction in Heileman’s definition of complexity. One could say this change makes
the pathways easier for students to get through, in a sense. Has it actually
been easier? I don’t know because most of our students graduate in four years
both before and after the change.
Here’s our biochemistry major. Technically the
required biology classes are independent of our chem/biochem offerings although
we’ve been advising the students to take Mol Bio Techniques before they take
Biochem Lab (usually in either semester senior year). Many of our biochem
majors delay P-Chem until their very last semester, and find it a miserable
experience. There are a variety of reasons for this including being behind in
the math/physics pre-requisites, trying to shelter one’s GPA when applying for
medical school, thinking that P-Chem 2 (stat them) is easier than P-Chem 1
(quantum), and others that I shall not mention as someone who teaches in the
P-Chem sequence.
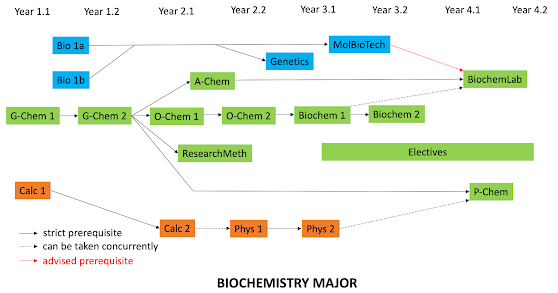
The longest path is once again five arrows starting
from G-Chem 1 and ending at Biochem 2 lecture. G-Chem 2 is still a hub, but not
O-Chem 2. Overall, our biochemistry major would have a lower complexity score
(per Heileman) compared to our chemistry major, but not by much. On average 80%
of our majors are in biochemistry with 20% in chemistry. Once again, there are
a variety of reasons for this: Many students are interested in health careers
and the biochemistry major fulfils the biology pre-requisites. Also, only one
semester of P-Chem is required rather than two. (Most of our students don’t
enjoy physics, and P-Chem is almost always rated as the hardest class,
whichever semester our biochem majors take.)
Before the redesign of both our majors, they were
more hierarchical. That is to say, they previously resembled similar majors at
large public universities (that also offer graduate programs). Interestingly,
small selective liberal arts colleges (SLACs) tend to have less hierarchical
majors – and therefore lower “complexity” – and they offer the option of taking
some additional electives if a student wants to be ACS-certified. Several SLACs
now only require one semester of P-Chem (so a student who wants an
ACS-certified degree opts to take a second semester). This allows a wider range
of electives and for students to create different pathways through the major
based on their interests. We offer a small range of electives because of other
constraints at our institution. However, if we’re willing to forego the
professional body certification, we could reduce the complexity of our majors. Several
SLACs also have an “O-Chem first” track and/or a single semester of “accelerated”
G-Chem. You can do this if you know the vast majority of your students have a
solid high school chemistry background (at an “honors” or AP-level, or even
just excelled at a single year of chemistry).
Math skills are also important for success in
G-Chem, and at many SLACs, students come in “calculus-ready”, i.e., they can
either comfortably slot into first-semester college calculus (and it’s a
breeze) or they go directly into a more advanced math course. That’s not the
case at my institution on average (although it is true for the better-prepared students).
It was interesting to see this as a key factor in Heileman’s presentation of
engineering pathways which have multiple math and physics prerequisites.
Pondering all this has made me wonder if we should consider scrapping our Calc
II and Physics II requirements and instead teach a “physics for chemistry”
class that then serves as a pre-requisite for P-Chem. Students would be less
shocked when they encounter P-Chem and they’d be happy to replace two courses
for one (including one less lab). I need to think about this a bit more
carefully before I consider proposing it to my department (and I might not).
One thing I have proposed is to re-envision the
O-Chem sequence so that O-Chem 1 leads to Biochem 1 lecture, so that only our
majors take both semesters of O-Chem. The majority of our students in O-Chem 2
(+ lab) and Biochem 1 are majoring in other departments but trying to complete
requirements for pre-health majors (e.g. medical school, dental school, vet
school). No, we haven’t made the change for a variety of reasons I won’t
discuss here, but we typically have 150 students in O-Chem 2, most of whom hate
being there. (And I know what it’s like to teach a class that students greatly
dislike being “forced” to take.) Over the years, we’ve seen the elite medical
schools no longer require O-Chem 2, but would like to see Biochem in a student’s
transcript. Not surprisingly, other medical schools are following suit.
(Roughly a third still required O-Chem 2 + lab, at least a couple of years ago
when I last checked.)
Our department has been entertaining the idea of
more flexibility and different pathways in our majors, and perhaps not being
wedded to our degrees all being certified by ACS or ASBMB. This might allow more
combinations and for students to choose things they are interested in. We might
be able to offer a greater spread of electives more often. And we might attract
more students to our majors, who are otherwise opting for other perceived “easier”
options that have recently been offered in other departments that “complete the
pre-health requirements”. This last point used to be something I was not concerned
about (because we were seeing year-on-year increases in our major which brought
its own challenges especially with our research requirement); but as I see
administrative moves towards thinking of budgets more atomistically. I now see potential
danger ahead that if we lose majors, we lose budgetary dollars, and one gets
into a downward vicious cycle – you can see this play out across the U.S. in
the humanities.
So how important is curricular complexity? I see
moves towards decreasing complexity, for a variety of reasons – complex,
multifactorial ones – and this blog post is already getting too long. One can
make many arguments for and against. There might be an optimal or sweet spot,
but it is likely to change over time as other factors vary. Perhaps the subject
of another blog post!












