What would you like non-science majors to know about the essence of chemistry? For chemist Sason Shaik at the Hebrew University, it would be the beauty of molecules and then anyone can learn how to construct them by imagining molecular LEGO. After teaching and refining his short-course in chemistry, he even wrote a book. Published in 2016, it is titled Chemistry as a Game of Molecular Construction: The Bond-Click Way.
As a computational chemist with some expertise in chemical bonding, I’ve read a lot of Shaik’s work. I particularly appreciate his versatility with making connections between valence bond theory and molecular orbital theory, showing that both are useful and complement each other. Some of these ideas have permeated my classes, in particular quantum chemistry and inorganic chemistry. But I’ve never met Shaik, and I didn’t realize his enthusiasm for teaching until reading his Bond-Click book.
Okay, I admit I skimmed many parts of the book – mostly because this was material I was familiar with. But I slowed down when reading his mock interviews that begin each chapter and pondered certain conceptual bits that related to my own teaching. It’s been a while since I’ve taught the non-science major chemistry course in my department (after the core curriculum was revised and the science requirement reduced). But I still teach general chemistry (for science majors) every year, so there’s much that is relevant. Here are some of my observations, in no particular order.
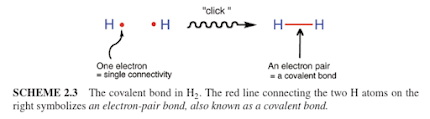
Shaik dives quickly into chemical bonding by starting with Lewis dot structures of atoms. A single unpaired electron on an atom can pair up with another single unpaired electron on a different atom – you “click” them together to make a bond, similar to clicking together two LEGO blocks. This is, in fact, the way I teach Lewis Structures in G-Chem 1, which is somewhat different from traditional textbooks. Maybe theoretical chemists think alike. He begins with two hydrogen atoms forming a H–H bond (see below) and then immediately introduces the bond energy curve. I actually introduce the bond energy curve first, so I agree with his approach that students should see this early!
But when do you stop clicking? Shaik introduces the Law of Nirvana, essentially the octet rule. I also talk about the octet rule, but emphasize that it is a rule-of-thumb for identifying stable molecular structures. Nirvana sounds like happy bliss, but one has to be careful not to go overboard with the (un)Happy Atoms story. In any case, he starts illustrating the construction of small simple molecules from their constituent atoms. Because he wants to get quickly to structures of organic molecules, he quickly moves on to molecular fragments. I agree with this approach in a non-majors chemistry course. One doesn’t need to introduce the “harder” parts of drawing complex Lewis structures at that level although he will touch on these aspects later. The point is to get students to see how interesting (biologically relevant) molecules are constructed from their parts. Shaik also peppers his lecture with interesting stories about these molecules!
What surprised me is that Shaik didn’t just build up linear and branched alkanes (which I would do), he also builds up cyclic alkanes using the same approach even starting with reactive molecules such as cyclopropane and cyclobutane. He then discusses tetrahedrane C4H4 (very unstable) and cubane C8H8, before building his way to dodecahedrane C20H20. I particularly enjoyed pondering this series of examples! After this he discusses diamond and graphite, something I also do in G-Chem 1, before talking about buckyball. I usually go further and discuss nanotubes – at which point the engineers in my class perk up and start to ask questions as I talk about constructing a “space elevator”.
Molecules can react with other molecules to form larger molecules. How so? According to Shaik, first you clack (by breaking a bond) and then you click (by forming new bonds). He illustrates this by discussing the formation of polyesters and polyamides in condensation reactions. He calls this the clack-click approach.
I liked how he approached hypervalent molecules by having students think about “electron-rich” molecules. He illustrates this with phosphoric acid (among other examples) which is then tied to ATP and DNA. Then eventually he gets to molecular shape by discussing the Pauli Exclusion Principle in a simple way without making use of quantum numbers. I found his approach effective. He even gets around discussing free rotation around single bonds versus cis-trans isomerism in C=C double bonds without resorting to hybridization or sigma versus pi bonds. Once again, I found his approach effective. It was a good reminder that a teacher can come up with simplifying yet effective explanations while skipping some of the background material. I’ve even considered jettisoning orbitals in G-Chem, although I’m unlikely to do so.
Ionic bonding is covered towards the end, after covalent bonds, and after introducing electronegativity and polar-covalent bonds. This is unlike many G-Chem textbooks that cover ionic bonding first. In a sense, ionic bonding is easier to “explain” since students don’t question why opposite charges attract (via Coulomb’s Law). It’s unclear why sharing a pair of electrons in a covalent bond provide an energy stabilization, at least at the G-Chem level, without going into quantum chemistry. Shaik only touches on metallic bonding briefly (as do I) but the way he emphasizes delocalized bonds is by fragment-building. He doesn’t push the model very far. I spent some time thinking about the effectiveness of the approach, but it might be tricky because crystal structure comes into play. One could just focus on the first column where the bulk metals have a body-centered-cubic structure. (I used to discuss band theory briefly in G-Chem but have axed it.)
Shaik sprinkles stoichiometry amidst discussing structures of molecules and chemical reactions. This is unlike a traditional G-Chem course where stoichiometry gets its own dedicated block of time. I tend to spend a little less time on calculational-problems in stoichiometry compared to some of my colleagues, mostly because I’m more focused on the conceptual parts. Maybe forty years ago stoichiometry-calculations were important to the chemist working in a lab, but in today’s working world for the chemist, I think this is much less important. Yes, I still cover it. Yes, there are exam questions on it. But it’s a smaller chunk of the topics I cover in G-Chem 1. In that sense, I liked Shaik’s approach, and it reminded me of how little emphasis I placed on calculational-stoichiometry when I regularly taught the non-majors course.
What I most got out of this book was Shaik’s enthusiasm! It made me feel excited about the next time I teach G-Chem 1 (next fall) and consider some new examples to use in my class. Seeing a different approach to teaching similar material is, in my opinion, something that we teachers need to do more regularly. I lament my not taking the time to discuss these sorts of teaching details with my colleagues in recent years. (I was much better at doing so in my earlier years as a teacher.) Shaik’s book was a reminder to me that I should get back to regular and substantive pedagogical discussions.


No comments:
Post a Comment